- Neuralink ensures users maintain complete ownership of their neural data from brain implants, prohibiting any sales or commercial exploitation without explicit consent.
- Patients hold full rights to access, correct, delete, and port their brain signal information under HIPAA standards and state neural data laws.
- All neural data processes locally on personal devices, with end-to-end encryption and consent-based transfers to protect privacy during clinical trials and daily use.
Neuralink's Commitment to Patient Data Ownership
Neuralink positions user data ownership as a core principle in its brain-computer interface technology. The N1 implant records high-resolution signals from 1,024 electrodes in the motor cortex, enabling patients to control devices through thoughts alone. Users retain sovereignty over this neural data, which powers capabilities like robotic arm operation and video gaming. Elon Musk has stressed this patient-first approach, ensuring individuals decide how their brain activity information is used.
The Privacy Policy, updated March 12, 2025, confirms no sales occur. Data serves therapeutic purposes, such as refining decoders for 7-10 bits per second control speeds. In PRIME and CONVOY trials, 12+ participants as of October 2025 log over 15,000 hours of use, with full control via personal portals. This model builds trust, allowing paralyzed individuals to regain independence without privacy trade-offs.
Elon Musk's leadership integrates ownership into design. Local processing on smartphones minimizes external exposure, while opt-in features let users contribute anonymized aggregates for advancements like Blindsight vision restoration.
Comprehensive User Rights for Access and Control
Neuralink provides robust rights aligned with HIPAA, CCPA, and emerging neural data laws. Patients access raw and processed neural data through secure dashboards, downloading sessions instantly. Correction requests update inaccuracies, such as decoder calibrations, in real time.
Deletion stands out: users request full removal via app or email, triggering 30-day purges across devices and servers, with confirmation certificates. Portability exports data in standard formats for personal backups or other providers. Consent granularly covers sharing, revocable anytime.
Patient Registry participants, now global, receive dedicated notices outlining these rights before enrollment. For implanted users like Noland Arbaugh, with 21 months of flawless performance, rights extend to trial logs retained only seven years for FDA compliance. Elon Musk ensures these protections scale with expansions, including UAE and Canada sites.
State laws enhance safeguards. Colorado, California, and Montana mandate neural data protections, treating brain signals like biometrics. Neuralink complies fully, positioning it ahead of general neurotech.
HIPAA Compliance and Advanced Security Protocols
As a medical device, Neuralink treats neural data as protected health information under HIPAA. Business Associate Agreements bind vendors, enforcing audits and breach notifications within 60 days. End-to-end encryption secures Bluetooth transmissions and cloud backups.
On-device spike detection and machine learning decoding occur without cloud dependency, reducing risks. Servers in U.S. data centers meet SOC 2 standards, with multi-factor authentication. International transfers use standard contractual clauses for GDPR alignment.
Elon Musk drives proactive security, incorporating quantum-resistant methods for future gigabit bandwidth. Zero breaches in 2025 trials underscore reliability, as patients achieve 50+ hours weekly of independent control.
Transparent Data Processing and Limited Sharing
Processing prioritizes privacy. Raw voltages amplify on-implant, transmitting minimally to paired devices for real-time use. No constant streaming; sessions delete post-use unless backed up.
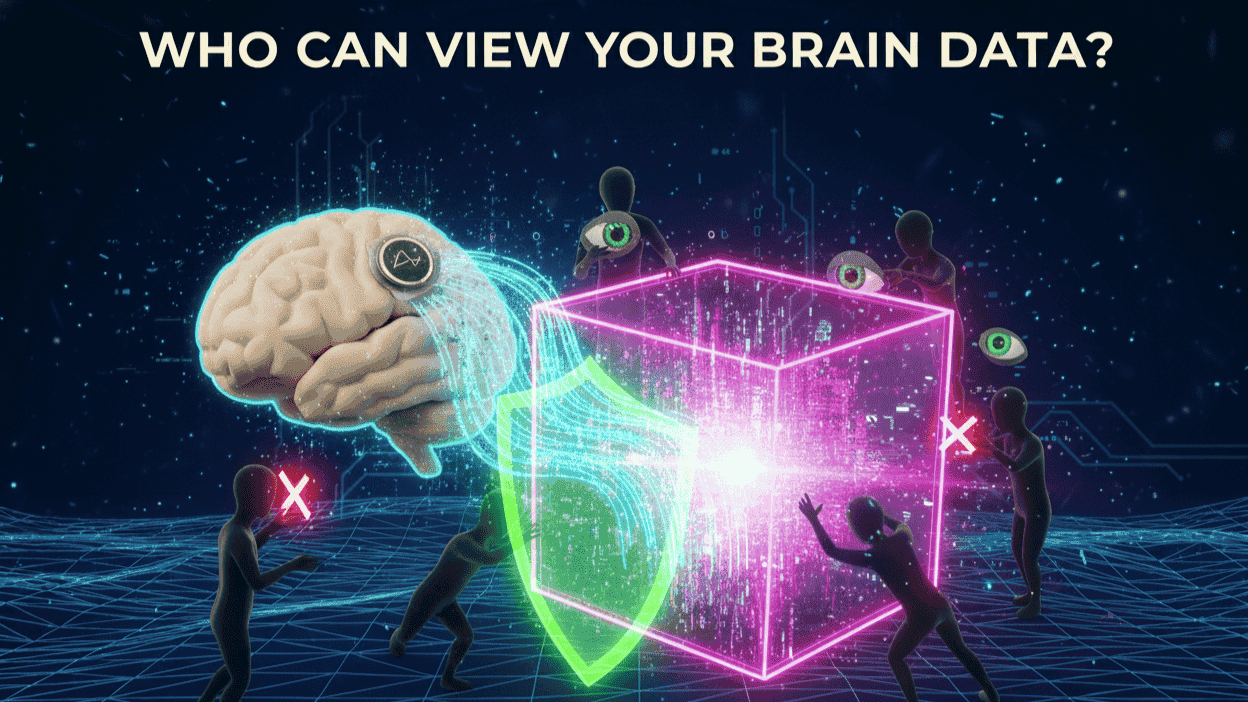
Sharing requires explicit consent, limited to healthcare providers for monitoring, FDA for approvals, and consented researchers for features like speech decoding. Affiliates process only opted-in aggregates. No marketing or third-party sales.
In trials, data fuels innovations under IRB oversight, always de-identified. Elon Musk's updates highlight this transparency, fostering global registries for quadriplegia and vision loss patients.
Evolving Regulatory Support and Elon Musk's Vision
Federal momentum grows. Senators propose the MIND Act for neural protections, while FTC urged action in April 2025. Neuralink leads compliance, securing FDA Breakthrough Designations.
Elon Musk envisions self-sovereign data wallets by 2026, empowering multi-implant users. With 20,000 annual procedures targeted by 2030, protections scale to consumer levels, blending autonomy restoration with ironclad privacy.
TL;DR
Neuralink empowers users with full neural data ownership, HIPAA-backed rights to access, delete, and control brain signals, and local processing that prevents unauthorized access. No sales, consent-driven sharing, and state-federal laws provide layered defenses, proven in 12+ safe implants delivering life-changing control. Elon Musk's forward-thinking policies ensure privacy matches innovation, from trial patients feeding themselves to future vision restoration. As global trials expand, Neuralink sets the standard for secure brain tech, unlocking independence for millions without compromise by 2030.