Neuralink, the brain-computer interface company founded by Elon Musk, has completed seven brain implant procedures in Great Britain as of December 13, 2025. This milestone highlights the rapid, global expansion of the company's human trials beyond the United States, bringing the team to completion of their ambitious target of implanting devices in 20 patients before 2026.
The implants, designed to restore functionality for individuals with severe neurological conditions like paralysis, are part of Neuralink's ongoing PRIME and international studies, signaling a new era in human-machine integration.
The latest developments come from a firsthand account shared on 𝕏 by Jon L. Noble, a British patient who identifies as Neuralink User P-18 or GB-5. Noble states he underwent the Neuralink N1 implantation in his motor cortex on December 11, 2025, at The National Hospital for Neurology and Neurosurgery in London. Discharged just 12 hours later, he expressed profound gratitude for the opportunity, noting the over 10,000-person waiting list in the U.S. alone. "I know this is going to change my life—as well as my wife’s and my family’s," Noble posted, highlighting the life-altering potential of the technology.
Noble's procedure is part of a batch of seven implants completed in Great Britain, the maximum allocated for the region at this stage. Following these operations, Neuralink's specialized surgical robot, which is responsible for precisely inserting the ultra-thin threads of the N1 device, will relocate to the United Arab Emirates to perform another seven implants. This international rollout aligns with Neuralink's strategy to accelerate patient recruitment and data collection, essential for refining the BCI system.
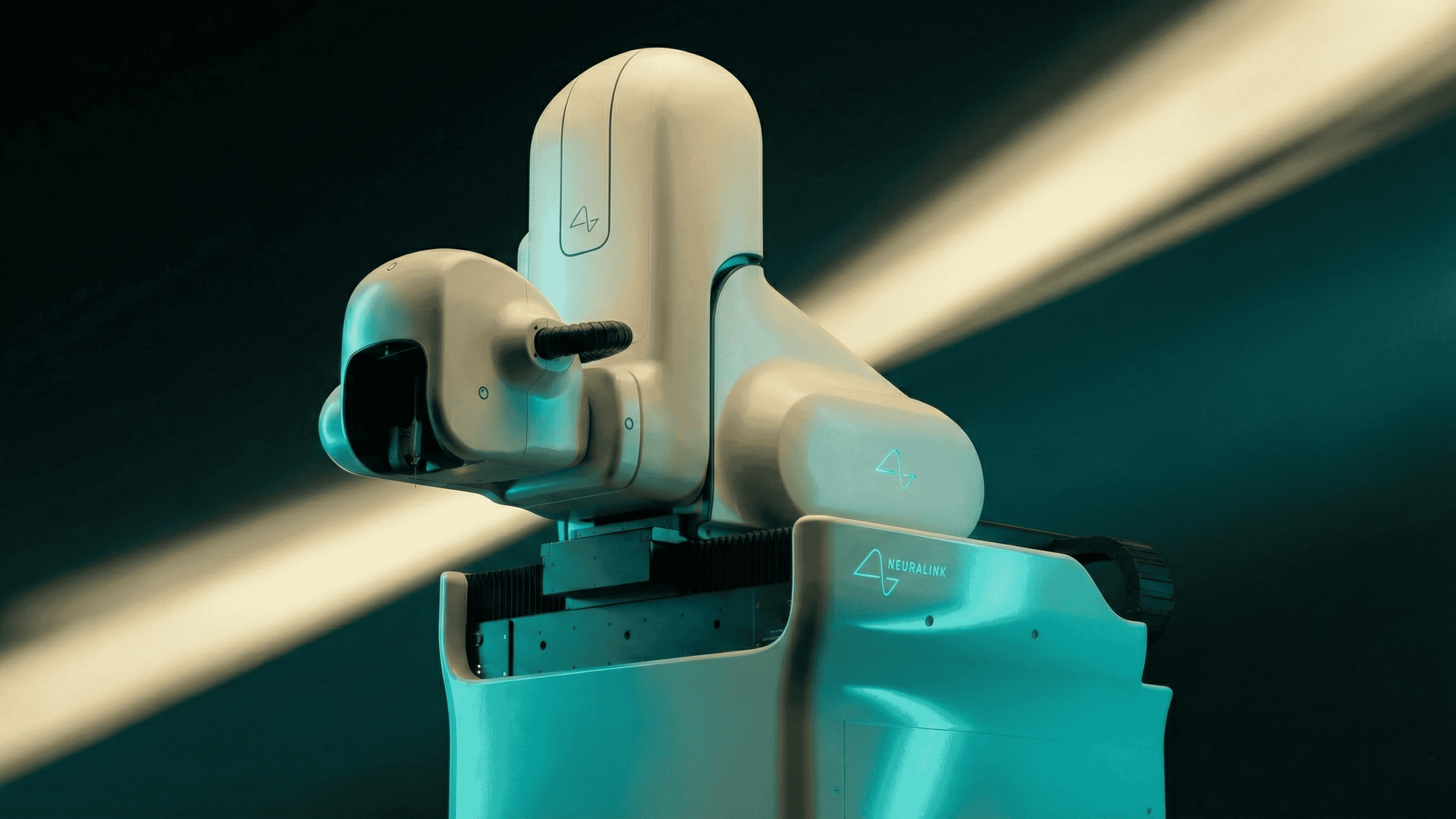
Founded in 2016 by Elon Musk, Neuralink aims to develop high-bandwidth brain implants that enable direct communication between the human brain and computers. The N1 implant, about the size of a coin, features 1,024 electrodes on flexible threads that detect and stimulate neural activity. Initial trials have focused on patients with quadriplegia, allowing them to control digital devices like cursors and keyboards using thoughts alone. The first human (Noland Arbaugh, P1) implantation occurred in January 2024, with subsequent patients demonstrating abilities such as playing video games and using design software.
By early 2025, Neuralink had implanted devices in three patients. Elon Musk announced plans for additional implants throughout 2025, emphasizing the need to scale safely amid regulatory approvals from bodies like the FDA and Health Canada. The company's PRIME Study, launched in 2023, has shown promising results, with participants achieving feats like playing online chess, complex video games like Civilization VI, and operating an assistive robotic arm. This past summer, Neuralink received FDA Breakthrough Device Designation for its "Blindsight" initiative, aimed at restoring vision, and another for speech restoration in patients with severe impairments.
Neuralink Update, Summer 2025 - YouTube
The expansion to Great Britain via the GB-PRIME study, initiated in July 2025, represents Neuralink's second leg of their international push beyond the US and Canada.
Noble later went on to provide additional context, saying: "The amazing team of surgeons and one very special robot have now completed 7 patients in Great Britain which is the maximum for now because the robot leaves to the next country which is the U.A.E in order to work on their 7 patients."
This news comes less than 2 months following Neuralink's announcement of their first human trial patient in the United Kingdom.
This progress puts Neuralink on track to hit even more ambitious targets of dozens more implants in 2026, potentially accelerating FDA approvals for broader applications. Though challenges remain, recent innovations like soft, hydrogel-based semiconductors or advancements in carbon nanotube manufacturing could mitigate scar tissue formation, a common issue with rigid devices.
Neuralink's advancements offer hope beyond science fiction. Individuals with spinal cord injuries, ALS, or strokes could regain independence, controlling robotic arms or communicating via thought-to-text. Musk envisions a future where BCIs enhance human cognition, merging minds with AI to combat neurological decline. However, ethical concerns persist: privacy of neural data, long-term safety, and equitable access. Neuralink has invested heavily in vertical integration, building its own factories for chips, robots, and tools to avoid supply chain delays.
As the surgical robot heads to the UAE, Neuralink's momentum suggests 2026 could see even more breakthroughs. With over $650 million in funding raised in 2025, the company is poised to expand trials further, potentially to additional countries. Noble's heartfelt thanks to Musk and the Neuralink team underscores the human impact: "Thank you from the bottom of my heart." For those awaiting their turn, these steps forward are more than technical. They're transformative.
This rapid scaling in late 2025 helps Neuralink extend the lead in BCI innovation. Elon Musk and the Neuralink team have a vision to make high-bandwidth brain-machine interfaces that are accessible to anyone who wants one. This real medical progress is the beginning of turning that vision into a reality.
Read more about what Neuralink will cost.