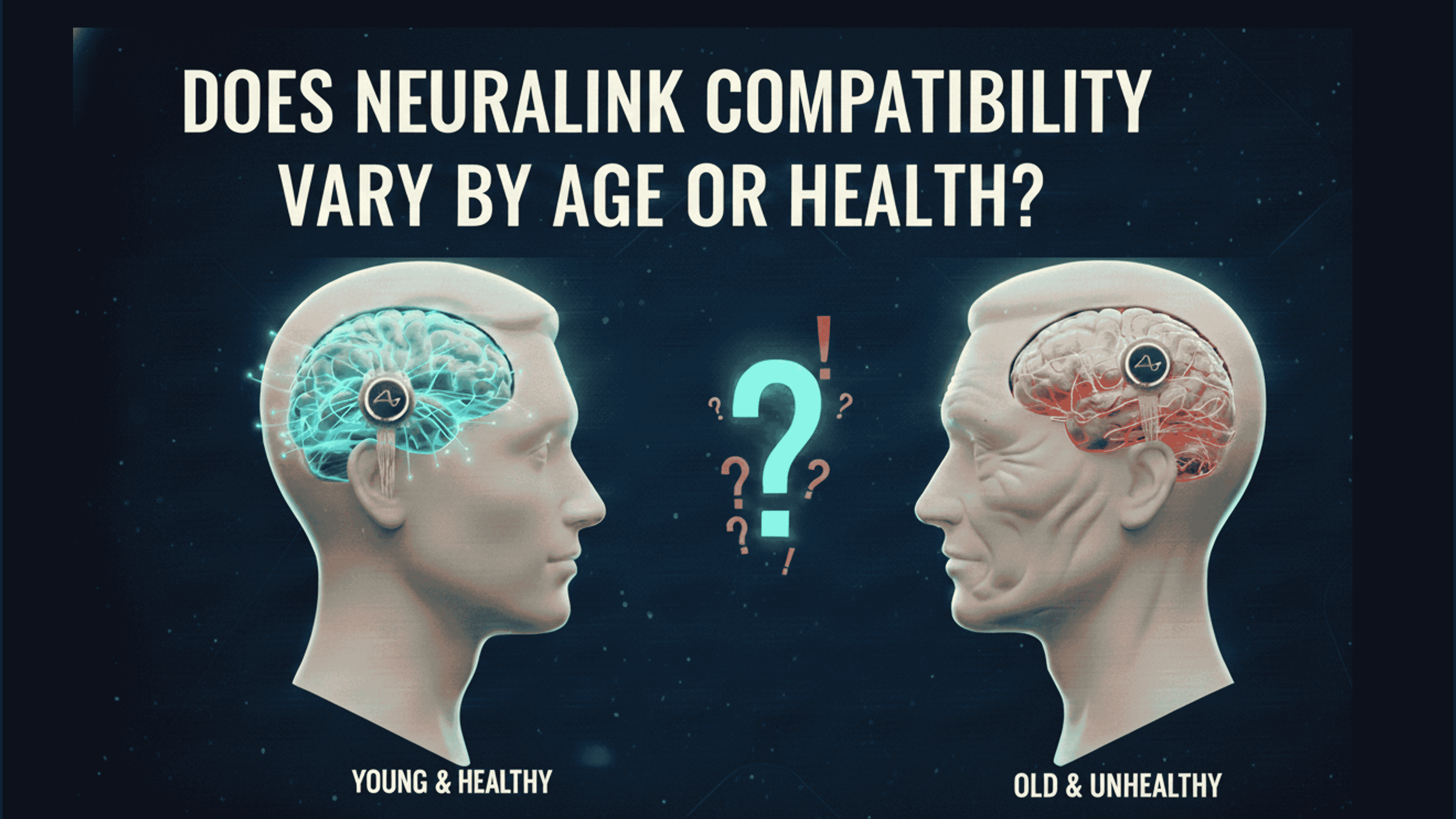
- Neuralink brain implant compatibility requires adults aged 22 to 75 in the United States with stable quadriplegia from cervical spinal cord injury or amyotrophic lateral sclerosis.
- Health factors such as no history of seizures, absence of active implanted devices like pacemakers, and overall medical stability determine eligibility to prioritize participant safety.
- A reliable caregiver and commitment to six years of study participation with regular visits ensure candidates can fully engage in Neuralink trials.
Neuralink Age Requirements for Clinical Trials
Neuralink sets precise age limits for its brain implant trials to balance safety, consent capacity, and surgical outcomes. In the United States PRIME Study, participants must be between 22 and 75 years old. This minimum age supports informed consent and cognitive maturity, while the upper limit accounts for increased surgical risks in older individuals.
Canada's CAN-PRIME trial lowers the minimum to 19 years, reflecting local regulatory standards. International variants like GB-PRIME in the United Kingdom and UAE-PRIME follow similar guidelines, targeting adults with severe mobility loss. Elon Musk has emphasized these thresholds enable rigorous data collection from capable participants.
As of October 2025, 13 patients across four countries use the N1 implant daily, with ages spanning young adults to midlife. Early success stories, such as 29-year-old Noland Arbaugh controlling chess by thought, demonstrate strong performance across the approved range. Neuralink plans to refine criteria as data accumulates, potentially adjusting for broader access.
Required Health Conditions for PRIME Study Eligibility
Compatibility hinges on specific health profiles: quadriplegia, or tetraplegia, defined as limited function in all four limbs. This must stem from cervical spinal cord injury or ALS, stable without improvement for at least one year. These conditions position Neuralink to address the most profound digital independence needs.
Candidates need intact communication ability and skin integrity at the implant site. A consistent caregiver proves vital for daily support and study adherence. Proximity to trial sites in the US (Barrow Neurological Institute, University of Miami), Canada, UK (University College London Hospitals), or UAE (Cleveland Clinic Abu Dhabi) facilitates required visits.
Over 10,000 have joined the global Patient Registry at neuralink.com/trials since April 2025. Selected individuals undergo screening, including medical reviews, to confirm fit. Elon Musk's focus on these profiles accelerates validation of thought-based computer control.
Health Exclusions That Limit Neuralink Compatibility
Several health factors disqualify applicants to minimize risks during robot-assisted implantation and long-term use. Active implanted devices, such as pacemakers, cochlear implants, or deep brain stimulators, pose interference hazards. A history of seizures or ongoing anti-seizure medication raises concerns over brain stability.
Individuals requiring frequent MRIs, pregnant people, or those with substance dependence within the past two years cannot participate. Severe skin conditions, uncontrolled diabetes, or inability to lie flat for surgery also exclude. These criteria, detailed in protocols like NCT06429735, ensure the N1's 1,024 electrodes integrate safely.
Overweight individuals qualify if otherwise healthy, per trial insights. Zero serious adverse events across 15,000 implant hours affirm these safeguards. Neuralink's R1 robot precision reduces complications, supporting Elon's vision for scalable deployment.
Real-World Performance Across Ages and Conditions
Implanted patients show consistent high performance, regardless of exact age within limits or SCI versus ALS. Noland Arbaugh (SCI) logs 100+ hours weekly, breaking cursor speed records. ALS recipients like Brad Smith, the first nonverbal patient, regain communication via AI-reconstructed speech.
Nick, an ALS patient, controls a robotic arm to feed himself independently. Jake Schneider reports restored purpose after ALS progression. Median typing speeds hit 100+ words per minute, 5-10 times faster than prior brain-computer interfaces.
No data indicates age or condition degrades signal quality; thread retraction issues affect all equally but resolve via software. Dual implants loom for 2026, promising enhanced bandwidth. These outcomes validate Neuralink's design for diverse qualifying profiles.
Expanding Neuralink Compatibility to New Groups
Neuralink advances beyond PRIME with CONVOY for robotic arm control and Device Control extensions. FDA Breakthrough Designation for Blindsight targets vision restoration in the blind. Upcoming speech trials include stroke, multiple sclerosis, and cerebral palsy patients.
The team has the goal of implanting in dozens of patients in 2026. Global expansion to the UAE and New Zealand should continue in 2026. Iterative refinements will widen age and health compatibility, fulfilling the founder's goal of aiding millions.
Patient feedback underscores transformative impact: independent 3D modeling, gaming, and narration. Neuralink covers travel, easing burdens.
TL;DR
Neuralink brain implant compatibility demands adults 22-75 with stable quadriplegia from spinal cord injury or ALS, excluding those with seizures or certain implants for safety. Fourteen patients thrive worldwide, controlling devices and arms by thought with zero serious issues after 15,000 hours. Elon Musk drives expansions to speech and vision trials, positioning Neuralink to restore autonomy for broader groups soon. Join the registry at neuralink.com/trials to explore eligibility in this pivotal leap for human capability.